南湖新闻网讯(通讯员 周吉隆 何海楠)近日,我校动物科学技术学院、动物医学院苗义良团队首次揭示了围着床期胚胎谱系分离中选择性自噬与细胞命运决定的调控机制。研究成果以“ATG7-mediated autophagy facilitates embryonic stem cell exit from naive pluripotency and marks commitment to differentiation”为题在Autophagy发表。
胚胎着床由精密复杂的信号调控和表观遗传调控所决定,其分子机理研究是动物繁殖领域发展亟需解决的核心问题之一。受精后,尚未植入子宫(pre-implantation)的胚胎中生成了数十个原始态(naive)胚胎干细胞,这些细胞具有分化成动物体内所有细胞的潜能。这种naive态干细胞的多能性是高度动态且连续发展的,当胚胎植入子宫后,naive干细胞很快迈出向特定细胞类型分化的第一步,变为始发态(primed)胚胎干细胞。naive-primed态过渡期间,naive态干细胞的染色质结构以及表观遗传发生了全局重塑以抑制naive态干细胞多能性基因的表达,激活早期分化基因,进而促进primed态胚胎的着床及发育。
作为一种重要的分解代谢过程,自噬以溶酶体降解和再循环途径维持多能性相关蛋白的适当水平和干细胞内稳态。目前的观点认为,特异性自噬调节因子局限于调控线粒体重构以促进体细胞重编程,以及通过降解功能紊乱的线粒体,降低细胞氧化应激来维持naive态细胞的多能性。然而,目前尚无研究揭示细胞自噬能否从表观遗传水平调控胚胎干细胞的多能性转换。
本研究构建了mCherry-GFP-LC3双荧光报告细胞系,利用体外胚胎干细胞naive-primed转换模型,通过电子显微观察及活细胞监测等技术阐述了naive态胚胎干细胞多能性转变过程中自噬通量的变化,证实了自噬对干细胞命运转变的决定性作用(图1)。
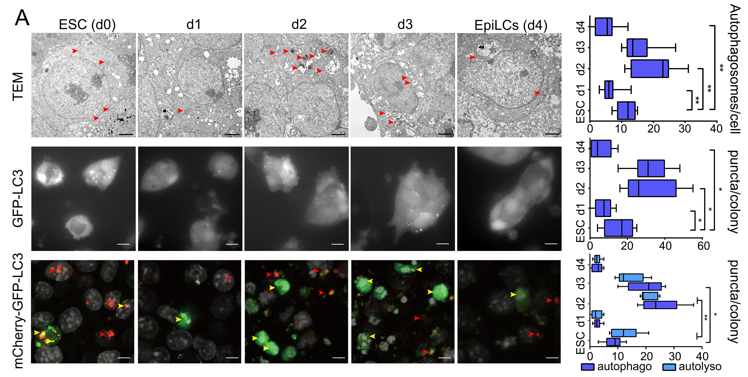
图1:naive-primed转化过程中自噬流的动态变化
基于RNA-seq和染色质转座酶可及性测序(ATAC-seq)联合生物信息学分析,本研究描绘了胚胎干细胞多能性转换过程染色质可及性动态变化规律,并发现自噬阻断条件下NANOG作为阻止多能性转变的屏障,一方面抑制分化相关位点的开放,另一方面维持naive多能性基因位点的开放以抑制多能性退出。此外通过染色质免疫共沉淀技术(ChIP)证明NANOG通过竞争性结合OTX2调控的特异性神经外胚层发育的相关区域来抑制神经元分化。通过CRISPR/Cas9构建的ATG7敲除小鼠模型,评估了自噬丧失会破坏围着床期胚胎的发育,进而导致新生小鼠神经发育异常及死亡。围着床期胚胎的异常发育是导致早期妊娠流产的主要原因,针对选择性自噬在围着床期胚胎发育中调控机制开发相关技术,对改善动物妊娠和构建naive态大动物胚胎干细胞具有重要意义(图2)。
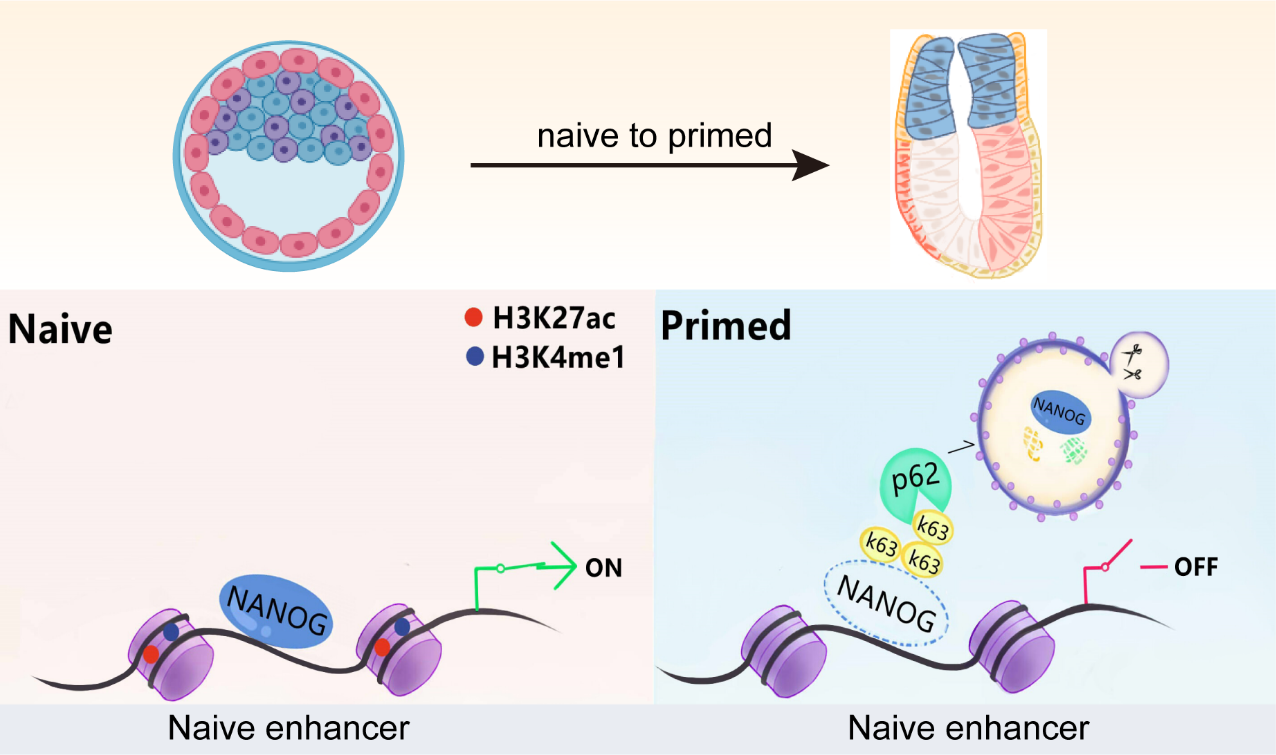
图2:细胞命运转换过程中选择性自噬参与的蛋白稳态调控及染色质变化
我院动科动医学院副研究员周吉隆和何海楠博士、张晶晶博士为论文的共同第一作者,我校苗义良教授为论文通讯作者。本研究受到了国家重点研发计划和国家自然科学基金的资助。
审核人:苗义良
【英文摘要】
Macroautophagy/autophagy is a conserved cellular mechanism to degrade unneeded cytoplasmic proteins and organelles to recycle their components, and it is critical for embryonic stem cell (ESC) self-renewal and somatic cell reprogramming. Whereas autophagy is essential for early development of embryos, no information exists regarding its functions during the transition from naive-to-primed pluripotency. Here, by using an in vitro transition model of ESCs to epiblast-like cells (EpiLCs), we find that dynamic changes in ATG7-dependent autophagy are critical for the naive-to-primed transition, and are also necessary for germline specification. RNA-seq and ATAC-seq profiling reveal that NANOG acts as a barrier to prevent pluripotency transition, and autophagy-dependent NANOG degradation is important for dismantling the naive pluripotency expression program through decommissioning of naive-associated active enhancers. Mechanistically, we found that autophagy receptor protein SQSTM1/p62 translocated into the nucleus during the pluripotency transition period and is preferentially associated with K63 ubiquitinated NANOG for selective protein degradation. In vivo, loss of autophagy by ATG7 depletion disrupts peri-implantation development and causes increased chromatin association of NANOG, which affects neuronal differentiation by competitively binding to OTX2-specific neuroectodermal development-associated regions. Taken together, our findings reveal that autophagy-dependent degradation of NANOG plays a critical role in regulating exit from the naive state and marks distinct cell fate allocation during lineage specification.
原文链接:https://www.tandfonline.com/doi/full/10.1080/15548627.2022.2055285

