南湖新闻网讯(通讯员 刘立虎)近日,我校资源与环境学院邱国红教授课题组在重金属污染场地土壤修复领域取得系列新进展,相关成果以“Highly efficient removal of Cu-organic chelate complexes by flow-electrode capacitive deionization-self enhanced oxidation (FCDI-SEO): Dissociation, migration and degradation”“Remediation of As-contaminated soils using citrate extraction coupled with electrochemical removal”和“Photoinduced self-organized precipitation in leachate for remediation of heavy metal contaminated soils”为题分别发表在Chemical Engineering Journal、Science of the Total Environment和ACS ES&T Engineering期刊。
土壤中重金属具有高毒性、环境持久性和弱迁移性,严重威胁生态环境和人类健康;尤其是针对重金属污染程度高的场地土壤修复技术研发迫在眉睫。各种物理、化学和生物方法(如电动修复、钝化、淋洗、植物修复和微生物提取等)已被用于降低土壤中重金属总量或生物可利用组分含量,以缓解其在食物链中的累积,而淋洗以其快速高效的修复速率已成为最常用的土壤修复方法之一。然而,化学提取剂的使用可能对土壤造成二次污染,同时产生含重金属的淋洗废水需进一步处理,增加了修复成本,这些不利因素限制了淋洗技术在土壤修复中的应用。
课题组先前研究表明环境友好的小分子有机酸(如柠檬酸和草酸等)可通过溶解、氧化还原和络合方式将土壤中重金属有效浸提到淋洗液中,其用于重金属污染场地土壤提取剂具有好的应用前景(Xiong Yang, Lihu Liu, Wenfeng Tan, Chengshuai Liu, Zhi Dang, Guohong Qiu*, Environ. Pollut., 2020, 264, 114745)。基于此结果,课题组进一步发展了小分子有机酸淋洗-电化学/光化学氧化联合技术用于重金属污染场地土壤修复。
首先,构建了流动电极电化学体系,并考察了其用于处理含有机酸络合态重金属土壤淋洗液的可行性及机理(图1)。结果表明流动电极电化学体系可高效去除模拟土壤淋洗液中有机络合态重金属。在流动电极电化学体系中,电场下较稳定的有机络合态重金属离子主要以带负电荷的形态迁移至阳极室;而亚稳态的有机络合态重金属离子则极易发生解离,并以游离态阳离子迁移至阴极室。带负电的络合态重金属离子迁移到阳极室后解络,有机小分子可被电化学及电化学介导生成的•OH氧化降解。在初始pH 3.5–5.5范围内,恒定电流10 mA时,模拟土壤淋洗液中络合态重金属(初始浓度1.0 mmol L−1)去除率接近100%。流动电极电化学体系也具有优异的稳定性,经5次连续运行及电极再生后,对模拟土壤淋洗液中络合态重金属的去除率没有明显降低。该研究证实了电化学吸附和氧化体系用于土壤淋洗液有机络合态重金属去除的可行性,相关结果发表在期刊Chem. Eng. J.。
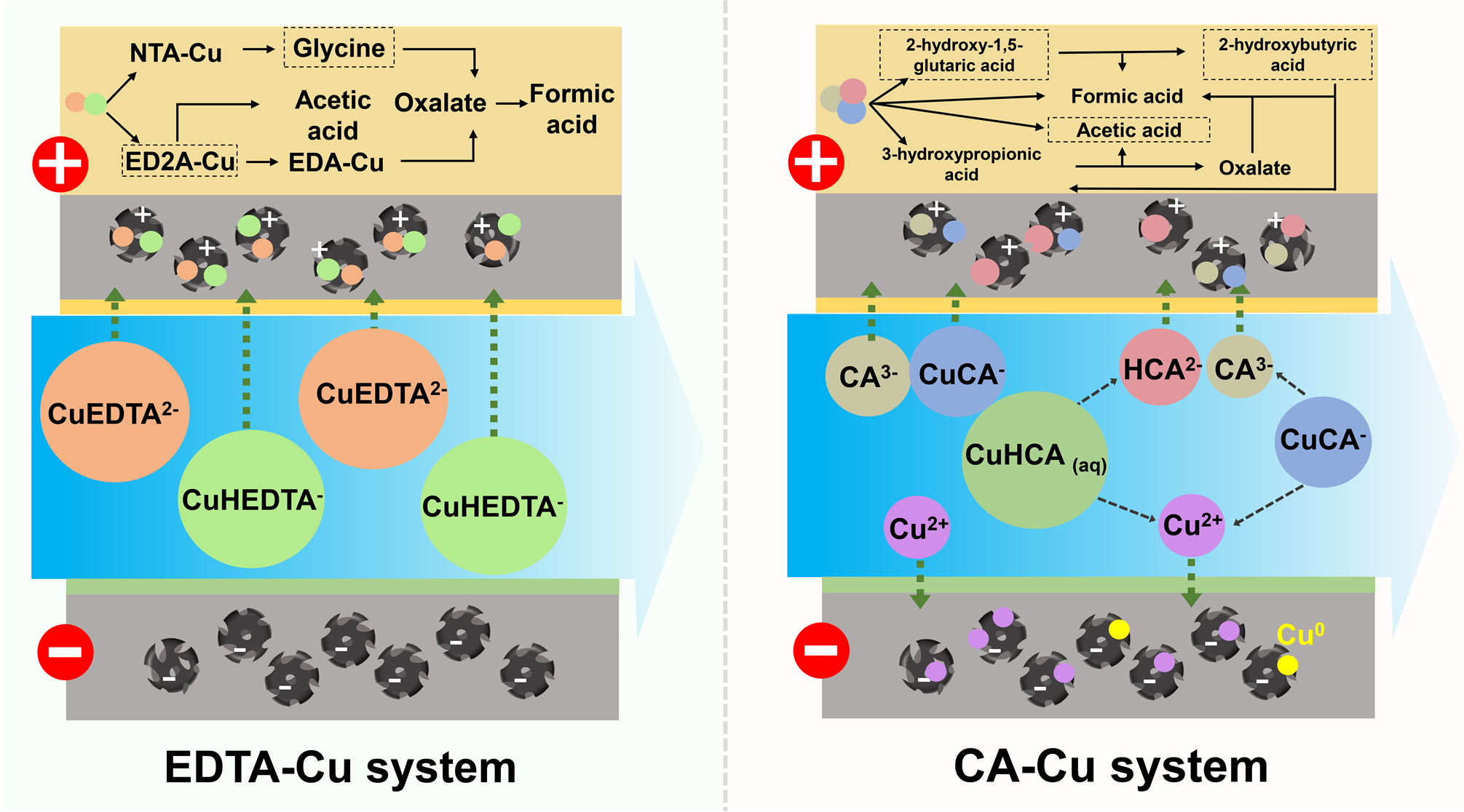
图1 流动电极电化学体系去除土壤淋洗液中有机酸络合态重金属离子示意图
其次,构建了小分子有机酸淋洗-氧化锰电极电化学氧化体系修复实际高浓度砷(As)污染场地土壤(图2)。经柠檬酸钠溶液淋洗和以水钠锰矿为阴极的不对称电解池中(槽压1.5 V)电解11天后,土壤中As总量和有效态含量分别由1980和242 mg kg−1降低至1260和152 mg kg−1,淋洗出的As可被完全去除。淋洗及电化学去除过程中,土壤中非晶质和晶质铁铝氧化物结合态As含量均显著降低,而其他形态存在的As变化不大。经三轮连续修复后土壤中As总量和有效态含量分别进一步降低至563和86 mg kg−1。柠檬酸盐通过溶解、配体交换及间接还原As(V)三种方式浸提去除了土壤中的As,而在电化学体系,淋洗液中As的去除主要归因于阳极上形成水铁矿和δ-MnO2及阴极上形成菱锰矿的吸附作用。相关结果发表在期刊Sci. Total. Environ.。

图2 小分子有机酸淋洗-氧化锰电极电化学氧化体系修复As污染场地土壤示意图
再次,发展了有机酸浸提与光辐照联合的方法用于污染场地土壤中多种毒性重金属的快速去除与回收(图3)。经柠檬酸提取后土壤中As和Cd(含量分别为1601和104 mg kg−1)的去除率达到73.0%和67.3%;从土壤中提取的Fe与柠檬酸结合形成Fe(III)-柠檬酸络合物,UV光照射下Fe(III)-柠檬酸络合物中配体向金属的电荷转移过程及伴随该过程产生的O2•−,导致柠檬酸氧化降解及重金属被沉淀或絮凝去除(60 h去除率高达97.0%以上)。小白菜盆栽实验结果表明经修复后土壤中重金属生物毒性降低,其中小白菜地上部分As和Cd降低率分别为22.0%和15.9%,根中As和Cd降低率分别为35.9%和58.6%。太阳光照射处理土壤提取液实验结果进一步证实了该方法潜在的应用性。相关结果发表在期刊ACS EST Engg.(Environ. Sci. Technol.姊妹刊)。
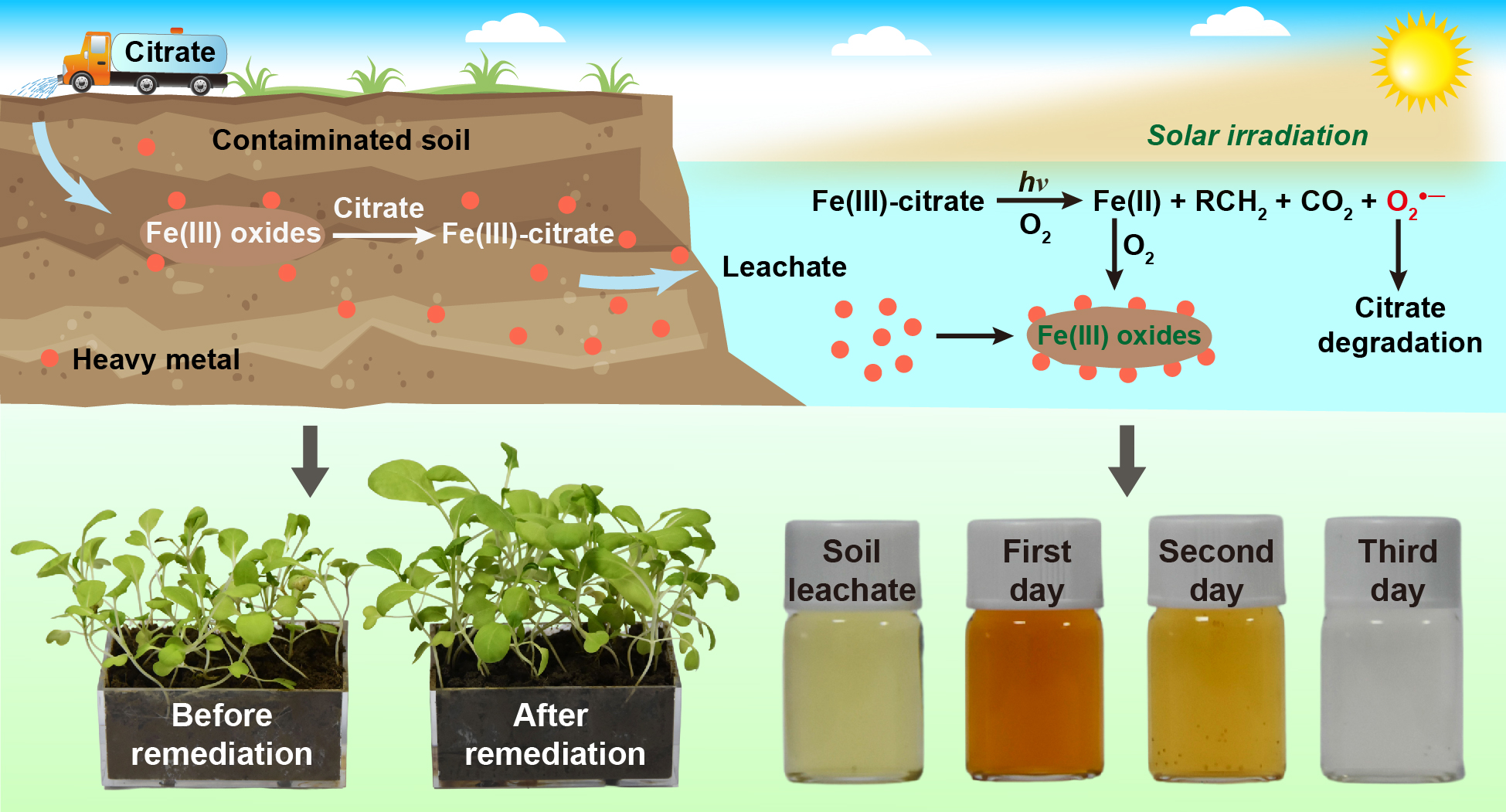
图3 有机酸浸提及光辐照结合的方法用于重金属污染场地土壤修复机理示意图
以上研究结果有望降低重金属污染场地土壤淋洗修复成本,同时扩展了电化学及光化学技术在重金属污染场地土壤修复领域中应用。
三篇论文(Chem. Eng. J.、Sci. Total. Environ.和 ACS EST Engg.)的第一作者分别为华中农业大学资源与环境学院硕士生尹浩宇、博士生杨雄和博士后刘立虎,通讯作者分别为博士后刘立虎、博士后刘立虎和邱国红教授(共同)、邱国红教授。华中农业大学谭文峰教授、曹梦华副教授、广东工业大学马金星教授和美国康涅狄格大学Steven L. Suib教授等在工作开展和论文撰写等方面提供了宝贵的意见和建议。以上研究得到了国家自然科学基金、国家重点研发计划、中组部“万人计划”科技创新领军人才及博士后创新人才支持计划等项目的资助。
【英文摘要】
(发表于Chemical Engineering Journal)
It is generally difficult to remove copper (Cu) from industrial wastewaters through traditional processes because Cu can form stable complexes with organic chelating agent. Here, the flow-electrode capacitive deionization-self enhanced oxidation (FCDI-SEO) system was applied to achieve highly efficient removal of two typical Cu-organic complexes (ethylenediaminetetraacetic acid-Cu (EDTA-Cu) and citric acid-Cu (CA-Cu)), and the underlying mechanism was also investigated. At the initial concentration of 1 mmol L−1, Cu removal efficiency reached 99.3% and approximately 100.0% respectively in FCDI-SEO treated EDTA-Cu and CA-Cu containing wastewaters after 75 min. Stable EDTA-Cu mainly migrated into the anode chamber as chelated Cu, while CA-Cu was easily dissociated and migrated into the cathode chamber as free Cu2+. After entering the anode chamber, the degradation rate of EDTA and CA was respectively 66.1% and 60.2%. Compared with the isolated closed-cycle mode, the short-circuited closed-cycle mode showed insignificant difference in Cu removal efficiency but higher energy efficiency. With pH increasing from 3.5 to 5.5, Cu removal efficiency showed little difference between the EDTA-Cu and CA-Cu system. With the addition of 180 mg L−1 extra Na+, Cu removal efficiency was about 80% and 90% in the EDTA-Cu and CA-Cu system, respectively. After operation for five cycles and regeneration of electrode, FCDI-SEO could still achieve stable removal efficiency. This work provides a new pathway for the removal of aqueous Cu-organic complexes.
论文链接:https://doi.org/10.1016/j.cej.2022.136811
(发表于Science of the Total Environment)
Arsenic (As) pollution of soils poses serious threats to the ecological environment. In this study, organic acid (citrate) washing and electrochemical removal (manganese oxide cathode) were combined to remediate highly As-contaminated soils, and the effect of voltage was investigated as well. Citrate could extract the As bound to iron and aluminum oxides and enhance As mobility by indirectly reducing As(V) to As(III) in the soils. During the electrochemical removal of As, the rhodochrosite produced from the reduction of birnessite at the cathode, the birnessite generated from the re-oxidation of released Mn(II) and the ferrihydrite formed from the hydrolysis of Fe(III) at the anode together contributed to the adsorption and fixation of As in the leachate. After three successive rounds of combined remediation by citrate (0.1 mol L−1) washing and electrochemical removal with birnessite electrode at 1.5 V, the As was totally removed in the leachate and the content of As bound to iron and aluminum (hydr)oxides was reduced by 84.2% in soils. Correspondingly, the contents of total and bioavailable As in the soil decreased from 1981.4 and 242.0 to 563.2 and 86.0 mg kg−1, respectively. The As removal efficiency from the leachate and soil increased with increasing voltage from 0 to 1.5 V. This study provides a new method for the effective treatment of As-contaminated soils.
论文链接:https://doi.org/10.1016/j.scitotenv.2022.153042
(发表于ACS ES&T Engineering)
Soil pollution by heavy metals has become an urgent environmental problem worldwide. However, existing remediation technologies have various deficiencies such as high energy consumption, long operation time, and secondary pollution, which largely limit their practical engineering application. Here, we propose an environmentally friendly method combining citrate washing and subsequent light irradiation for rapid and simultaneous removal of toxic heavy metals from soils. At initial concentrations of 1601 and 104 mg kg–1 in the soil, the As and Cd percentage removal reached 73.0% and 67.3%, respectively. The ligand-to-metal charge transfer in Fe(III)-citrate complexes and formation of reactive intermediate HO2•/O2•– contributed to the citrate degradation and high-efficiency heavy metal removal (above 97.0% after 60 h) via coagulation and precipitation in the leachate under ultraviolet irradiation. For the soil with a low content of Fe, the photochemical removal efficiency of heavy metals could be significantly improved by the addition of Fe3+ in leachate. Pakchoi cultivation showed an obvious reduction in biotoxicity of the contaminated soil after remediation. Solar irradiation experiments demonstrated the potential applicability of the green and efficient approach for remediation of heavy metal contaminated soils.
论文链接:https://doi.org/10.1021/acsestengg.1c00483
审核人:邱国红

