南湖新闻网讯(通讯员 聂春红)8月2日,我校水产学院高泽霞教授团队联合中国科学院南海海洋研究所林强研究员团队在鱼类肌间刺方面的研究成果以“Single-cell transcriptomes and runx2b−/− mutants reveal the genetic signatures of intermuscular bone formation in zebrafish”为题在National Science Review期刊发表。研究明确了鱼类肌间刺形成的关键细胞群,鉴定到调控鱼类肌间刺发育的关键基因runx2b,揭示了runx2b调控鱼类肌间刺形成的分子机制。
世界主要养殖鱼类都含有一定数量的肌间刺,特别是我国主养的青草鲢鳙鲤鲫鲂等大宗淡水鱼类,食用时极易出现卡喉咙的危险,严重影响了其产品的经济价值。高泽霞教授团队前期研究已利用转录组、蛋白组、小RNA组、全转录组等组学构建了鱼类肌间刺发生发育的组学资源库,并筛选到了关键的信号通路和候选功能基因,但肌间刺形成的关键调控基因尚不明确。
该研究首先利用单细胞转录组测序技术(scRNA-seq)定义了野生型斑马鱼肌间刺所在区域的18个细胞类群,筛选到了肌间刺形成显著相关的肌腱祖细胞、肌腱分化细胞、成熟肌腱细胞和成骨细胞类群。细胞拟时序分析结果推测肌间刺是由肌腱祖细胞经肌腱分化细胞,再分化为成骨细胞而形成的。根据基因在细胞轨迹上的表达模式,筛选到10个与肌间刺形成密切相关的功能基因,并在斑马鱼上运用CRISPR-Cas9基因编辑技术分别构建了基因突变品系。最终发现runx2b突变斑马鱼肌间刺完全缺失,且其个体生长、其它骨骼单元形成、肌肉脂肪酸和氨基酸含量等性状均未受到显著影响。scRNA-seq分析同时揭示了runx2b突变体斑马鱼的肌间刺发生区成骨细胞数量的显著减少,在个体和细胞水平明确了TGF-β/BMP signaling pathway在肌间刺形成过程中的重要调控作用。
此外,该研究团队在经济鱼类团头鲂(武昌鱼)上运用CRISPR-Cas9基因编辑技术构建了runx2b基因F0代突变体,发现runx2b突变体团头鲂的肌间刺发育亦受到显著影响,部分个体肌间刺数目减少30%以上,表明runx2b基因对经济鱼类肌间刺形成亦具有重要调控作用。鉴于团头鲂性成熟时间为2年,团队拟在后续研究中繁育获得纯和突变品系,以期获得肌间刺完全缺失的团头鲂新种质。
我校水产学院博士后聂春红、副教授万世明、博士生陈宇龙为论文共同第一作者,水产学院高泽霞教授和中国科学院南海海洋研究所林强研究员为论文共同通讯作者,水产学院王卫民教授参与指导了本研究,比利时根特大学Paul Witten教授与Ann Huysseune教授,巴西莫吉达斯克鲁兹大学Alexandre Hilsdorf教授等参与了本项研究。该研究得到国家大宗淡水鱼类产业技术体系、湖北洪山实验室、国家自然科学基金、中国科学院前沿科学重点研究等项目的支持。
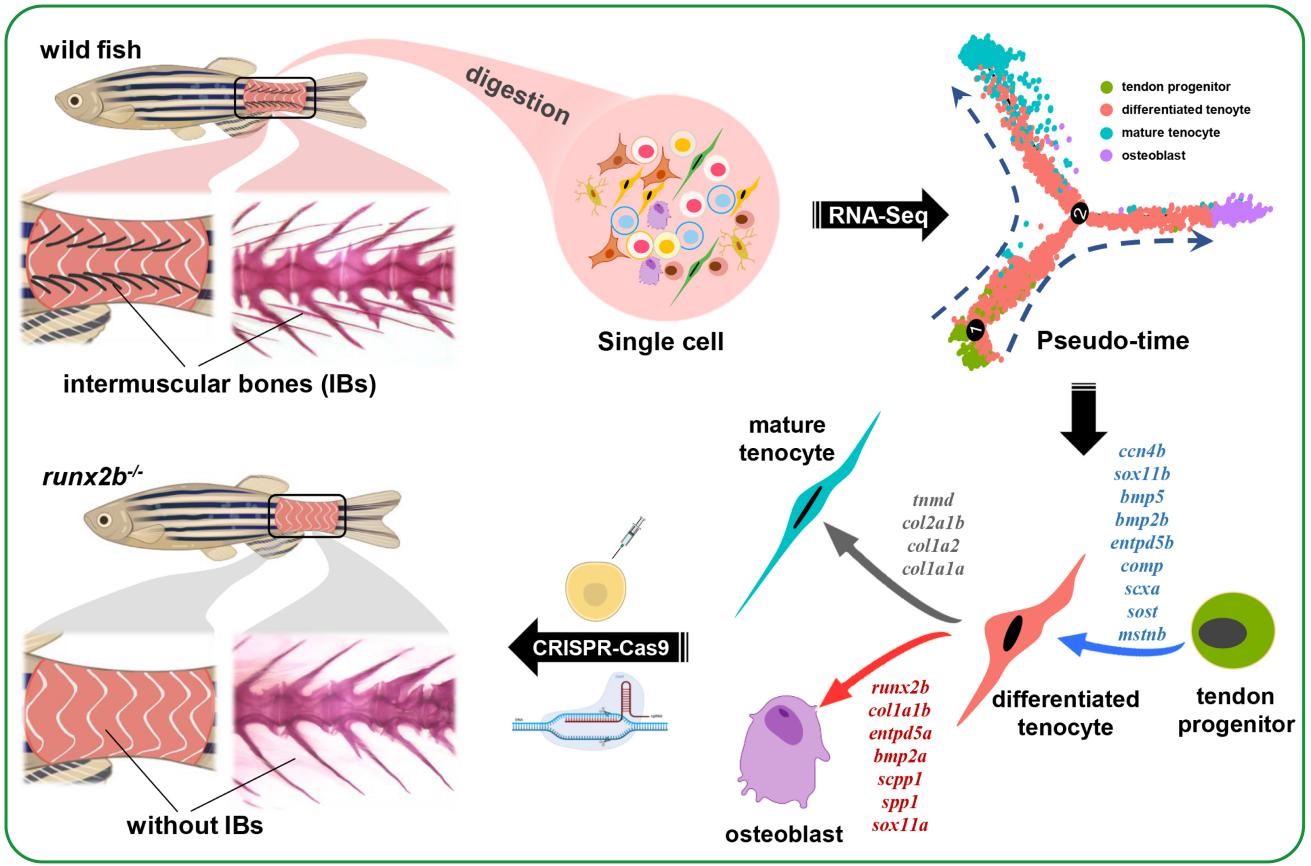
图1 论文摘要图
审核人:万世明、高泽霞
英文摘要:
Intermuscular bones (IBs) are mineralized spicules, present in the myosepta of many, but not all teleost species. IBs are often small and sharp, and they consequently limit how the fish can be processed, cause injury or trauma if lodged in consumers' throat or mouth and therefore affect the appeal of the fish to many consumers. The development of IBs in teleosts is still not fully understood and the molecular basis of IB development remains to be established. Here, the characteristics of IB tissue is evaluated based on single-cell transcriptomic in wild-type zebrafish. The analysis defined 18 distinct cell types. Differentiation trajectories showed that IBs are derived from tendons and that a core tendon-osteoblast cell lineage is related to IB formation. In particular, the functions of 10 candidate genes were evaluated via CRISPR-Cas9 mutants. Among those, runx2b−/−mutants completely lost IBs, while swimming performance, growth, and bone mineral density were not significantly different form runx2b+/+ zebrafish. Comparative scRNA-seq analysis in runx2b−/− and runx2b+/+ zebrafish revealed the role of osteoblasts in IB formation. In addition, differentially expressed genes were enriched in TGF-β/BMP pathway after runx2b deletion. This study provide evidence for a crucial role of runx2b regulation in IB formation. Genetic breeding can target runx2b regulation and generate strains of commercial fish species without IBs, which can improve the safe consumption and economic value of many farmed fish species.

